
Aquatic Habitats - Lakes et al.
Take a Tour of Lake Habitats Take A Tour of Wetland Habitats
When considering aquatic habitats, many variables come to mind. Obviously, there is a continuum of salinity ranging from essentially distilled water at glacier faces and high mountain streams, to other freshwaters, to estuaries where fresh and salt waters mix, to oceans, to hypersaline environments such as the Great Salt Lake. Current is another factor; water may be still and stagnant, or flow in currents of various velocities. Currents may be unidirectional, such as in streams, or multidirectional, such as when waves wash across a beach. Aquatic habitats may occur in open water, or they may be associated with the bottom of the body of water, and both will be affected by the mechanical and chemical makeup of the local geology. All sorts of daily and seasonal temperature regimes can be expected. Aquatic habitats vary in the amount of light they receive and range in size from tiny pools at the base of a plant to the Pacific Ocean in size. We'll start our discussion of freshwater lentic habitats by comparing them to marine habitats:
The obvious difference when comparing these two extremes is the salinity of the water, and the differences associated with that salinity. One obvious consequence of the difference in salinity is the change in osmoregulatory strategy that must take place. Many organisms in salt water are osmoconformers, essentially isotonic in relation to the seawater, although they may regulate certain ions at levels different from those of the surrounding ocean. A fair number of marine organisms are hypotonic in relation to the seawater and must therefore actively take up water to replace that they lose to the seawater. Of course, in taking on that water, they usually take in too many ions, and they must have some mechanisms for expelling those ions.
Organisms in freshwater have the reverse problem. They tend to take on water from the environment, and, in expelling the excess water, may lose important ions. Since freshwater is too dilute to make a good cytoplasm, it is no surprise that all freshwater organisms are hypertonic to the environment and that in consequence they must be active osmoregulators. Most have some mechanism to pump ions into the body. Of course, the whole problem of osmoregulation can largely be avoided by an impermeable outer body; but this in turn makes O2 uptake from the surrounding medium impossible. The respiratory surfaces thus become major sites of both ion and gas exchange. The linings of the gut and the kidneys also become important sites of ion regulation both in freshwater and in those marine species which are osmoregulators. Only two major groups of organisms with aquatic representatives do not depend on water for oxygen uptake. Both of these groups, the insects and the amniote vertebrates (turtles, snakes and lizards, crocodilians, birds, mammals) evolved on land and breath atmospheric air (some insect larvae take up O2 directly from the water and these comments do not apply to them). Their bodies are largely impervious to water or ion exchange, yet even in these groups we commonly see adaptations similar to those in groups with more intimate contact with the water. For example, both sea birds and marine turtles have salt glands near the eyes which eliminate ions from the body, and marine mammals have highly efficient kidneys.
It can be assumed that the salt content, high or low, of a body of water has relatively little impact on the taxa which are found there, since virtually all taxa have representatives in either freshwater or marine or even hypersaline environments. The only real difference in taxa composition of communities appears when comparing terrestrial and marine habitats, which seem to be the most ecologically distinct (despite the common environmental problem of water loss faced by organisms in both these habitats). Marine habitats are essentially devoid of insects and flowering plants, two groups which coevolved on land and do not seem inclined to move into marine habitats. Competition from organisms already there is often cited as a reason, but it is more likely that there has simply not been enough time for them to evolve into marine niches. In addition, both of the terrestrial groups have life cycle adaptations that are not well-suited for open aquatic habitats in any event. Going the other way, many marine species have not moved successfully onto land. Most cited are the crustaceans, and again, the usual reason given is competition from insects, but it is more likely that crustaceans have not moved onto land simply because they have not evolved breathing mechanisms that are effective on land; the same could be said for the echinoderms. In addition, many marine species have larval stages that could not exist on land even if the adult form could. It seems obvious to say that fish haven't made the transition to land, but this would not be accurate; fish did move onto land over 300 million years ago (MYA); we call their descendants amphibians, reptiles, birds and mammals.
Another difference between freshwater and saltwater, besides the ion concentration, is the density. Although there are probably other explanations, it is true that the largest organisms which have ever lived have been marine. Whether this is due to the greater support offered by dense seawater, or due to the fact that marine systems have larger volumes, or due to some other factor is not clear.
The depth of freshwater systems is usually much shallower than that of marine systems. As we saw earlier, depth is not a critical factor as long as the bottom of the body of water is above the LCP (light compensation point). Many freshwater bodies of water, including both rivers and lakes, have the bottom well within this range. Remember, though, that freshwater is highly susceptible to turbidity caused by soil erosion, thus the LCP might be artificially raised above the bottom. Marine systems, at least those away from the coast, are not usually affected by turbidity. The deepest freshwater lakes are about 2.7 km deep (and this occurs only in Lake Baikal, Siberia); oceans are up to 12 km deep, and the average depth of the oceans - or even the shallow part of the oceans, the continental shelf - is much greater than the average depth of freshwater and is almost always below the LCP.
Temperature relations in marine systems as opposed to freshwater systems are again largely dependent on the relative size of the systems. Generally, the larger marine systems show virtually no diurnal temperature shifts, and very small seasonal ones. On the other hand, small freshwater habitats may experience daily shifts in temperature of over 30 K, and pronounced seasonal temperature changes exist even in bodies of water as large as the Lauretian Great Lakes. Oceanic systems are a large part of the global weather system, which in general moves heat from the warm equator to the cooler poles. Oceanic areas exposed to currents involved in this heat transfer may be much warmer or cooler than would be expected due to their latitude alone, for instance, consider the relative warmth of the ocean near Britain due to the Gulf Stream, or the cold water off the southern California coast.
In a lake, water can pile up at one end of the lake due to a consistent wind from one direction. When the wind stops, the piled up water will flow back to the other end. This is known as a seiche, and can easily be replicated in a bathtub by using your body to pile up the water at one end. After the water sloshes back, it usually overshoots by some margin, and ends up piling up at the other end, the sloshing back, etc., for some time (Fig. 1). Of course, each successive slosh is less than then previous one, and in a lake, the only really noticeable differences occur during the wind (the set-up) and during the first seiche. Seiches may become quite complicated in lakes stratified by temperature or salinity differences as the lower levels will slosh at a frequency different than the upper ones. This sloshing of the lower levels is known as an internal seiche.
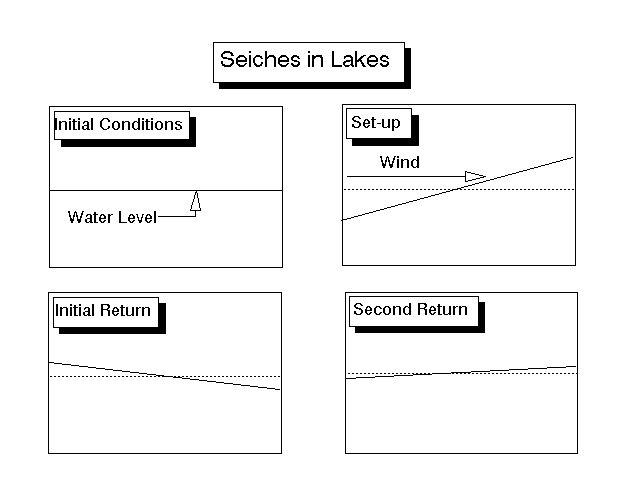
Figure 1. Action of a seiche. In the upper left drawing, the lake is at its normal level. In the upper right drawing, a consistent wind piles water up at one end of the lake. In the lower left, the wind stops and water flows back in response to gravity. Momentum of the flowing water causes the return flow to be greater than the original volume of water in that part of the basin, resulting in higher than normal levels. Gravity acts on this elevated water and causes it to flow back to the far side of the lake in the lower right picture. The oscillations decrease in magnitude each time. Vertical scale greatly exaggerated. For more on the physics of water, click here.
Aside from the comparison between marine and freshwater systems, we should also make a distinction between lotic, or running waters, and lentic, or still waters. These distinctions, of course, make sense only in freshwater; the entire ocean is lentic, although small areas swept by strong currents might somewhat resemble a lotic system. We will examine lentic systems first.
Lentic systems form when water is trapped at a level above sea level (or below sea level without an outflow). We call large lentic systems lakes, smaller ones ponds. Ponds and lakes form when something blocks a stream, such as a mudflow, avalanche (cirque lakes), beaver, or human. Even more lakes form in natural depressions such as old volcanic craters (caldera lakes), earthquake rifts (graben or rift lakes), sinkholes, or low-lying areas. Glaciers may scoop out depressions (the Lauretian Great Lakes), or leave behind large blocks of ice in the soil (till) they deposit as they retreat. The block of ice creates a void (hole) in the till, and as the ice melts it fills the hole (kettlehole lake). Lakes also form on riverplains when a bend of the river is cut off (oxbow lakes)
|
Cirque Lake |
Beaver Pond |
Reservoir |
|
Glacial Lake (Superior) |
Kettlehole Lake |
Oxbow Lake |
|
|
Many characteristics of a lake are consequences of its basin and its catchment. The basin, as explained above, determines the lakes size, shape and depth. For instance, glacial lakes tend to be very deep, while beaver ponds are shallow. The catchment is the area of ground surrounding the lake that contributes water to it. All of the streams and/or rivers upstream of the lake (feeders), and the water they drain from the surrounding land contribute to the catchment. Subsurface water (groundwater) may also contribute water to lakes. The nature of the soil and rock in the catchment will have a great impact on the water chemistry of the lake. For instance, glacial lakes are often surrounded by swampy land (bogs), and these bogs produce large amounts of moss, which sinks under the water and begins to decay. Because it decays slowly, under anaerobic conditions, acid conditions prevail, and the water in a glacial lake is often stained with dark brown humic acid. Lakes in areas with lots of limestone are often very alkaline. Land use within the catchment will also affect the water in the lake; farming the land will contribute soil (eventually filling in the lake), fertilizers and pesticides; cities will mean rapid runoff after storms because water flows off pavement rather than sinking in, and oils, salt, etc. from city streets will enter the lake.
Another important concept in lentic systems is residence time. Residence time is simply the average amount of time water spends in the lake. It can range from minutes to years; for instance, in Lake Erie it is about 2.5 years. Another way of looking at residence time is to empty the lake and see how long it takes to fill. This obviously is not practical for most lakes. The residence time affects many things, including water chemistry. If, for instance, a dangerous chemical is accidentally dumped into two lakes, the lake with the shorter residence time will be able to flush out the toxin more quickly. In certain saline lakes, where there is no outflow, residence time may be short for the water (due to high temperatures and rapid evaporation), but no flushing will occur.
Because there is no single, directional flow in a lentic system, stratification may occur. Stratification is the horizontal partitioning of a lake into strata, layers of water that do not mix. The basis for stratification is usually density differences induced by temperature, however, other factors that change density, such as salinity, also may be responsible. In a typical lake in a temperate climate, stratification normally manifests itself in the formation of two layers, a warm upper epilimnion, and a cool, lower hypolimnion.
It is perhaps easiest to see how stratification works by examining a typical temperate lake throughout the year. In the summer, the sun warms the upper levels. Since warm water is less dense, it tends to float, and the warm water concentrates at the surface. Between the warm upper water layer, and the cool, dense water below, there is a zone of rapid temperature change, the thermocline (Fig. 2). The thermocline is not necessarily a sharp plane, often the zone of temperature drop may extend for several meters. The depth and thickness of the thermocline are affected by the lake basin, wind, insolation, and other factors. Obviously, shallow lakes may warm all the way to the bottom and not exhibit a thermocline. In other shallow lakes, the bottom layer may be fed by springs; in such cases the thermocline may be quite abrupt; you may have experienced such thermoclines when swimming in a pond.
The division of the lake into these two zones has a profound effect. The epilimnion is mostly above the LCP for many organisms, and these organisms quickly use up all the nutrients as they photosynthesize. The wind may mix the upper layer, insuring ample O2 throughout, but the hypolimnion will not be mixed because the density difference is too great for the wind to overcome. The hypolimnion, below the LCP for most organisms, will become anoxic. As organisms in the epilimnion die, they will fall to the hypolimnion, carrying essential nutrients with them, and, in the hypolimnion, even more O2 will be used in their decay, exacerbating the O2 deficit there. Anaerobic respiration will become the rule, and bacteria which can survive under these conditions may add things like methane and H2S to the water, making it acidic and smelly.
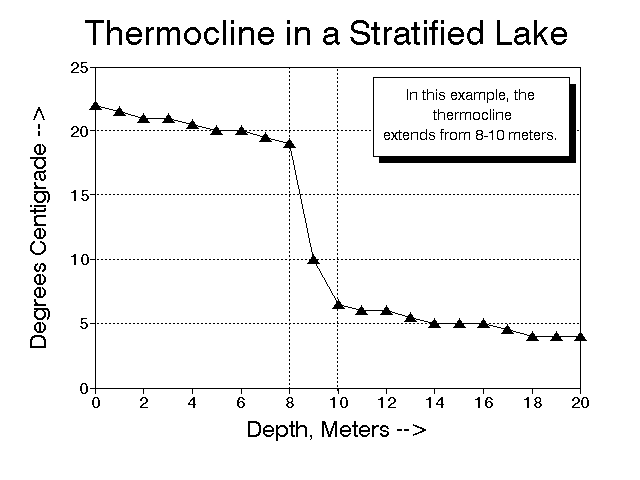
Figure 2. Graph showing a temperature profile of a stratified lake. In this example, warm surface waters (epilimnion) persist to a depth of about 8 meters, where they meet the cold, dense water of the hypolimnion. The zone where the temperature changes rapidly, from 8 to 10 meters, is the thermocline.
Some organisms take advantage of the differences between the hypolimnion and epilimnion. Because of the lack of O2, most fish avoid the hypolimnion, and thus it becomes a refuge from fish predation for the organisms which can live there. Chironomus larvae (which will grow up to be midges, which resemble mosquitoes but don't bite) have hemoglobin very similar to ours (2 subunits rather than 4); they can absorb O2 from the water even at low O2 levels. The Chironomus larvae (a.k.a. bloodworms) feed on bacteria, which, as mentioned above thrive on the rain of detritus (decaying organic material) from above. Chaoborus larvae (the adults of which look a lot like midges and mosquitoes, and also don't bite) are predators themselves; these bizarre-looking, transparent (they are called phantom midges), larvae hide at the bottom during the day, then rise up to the epilimnion at night to feed on plankton, presumably when capture by fish (which often rely on sight to capture prey) will be less likely. Chaoborus larvae make their vertical migration with the aid of small air sacs in their bodies; they can add or subtract air from these sacs to alter their density. Such diurnal vertical migrations are even more common in marine systems.
As summer ends, the amount of heat gained by insolation during the day will be less than the amount lost by radiation of heat at night. The surface waters will radiate heat to the atmosphere at night, cool, become more dense, and sink. Eventually, the whole water column will be at the same cool temperature. At this point, any wind pushing on the surface water can cause the water to be set in motion, and the water from the bottom is free to mix with that on the surface. This mixing is an important time for life in the lake. It allows the nutrients which have accumulated on the bottom to come to the surface, and it also allows O2 to reach the bottom of the lake.
Stratification of a different type will occur when the lake freezes in the winter. Here the surface water (ice) is much less dense than the other water and thus floats on top. Ice cuts down on O2 exchange, but this is not as critical in the winter when the cold temperature has slowed down the metabolic rates (and thus O2 demands) of most of the organisms in the water. Still, long ice covers may cause fish kills. Snow cover on the ice may drastically reduce light levels also, but, most importantly, the ice and snow cover reduce heat loss from the lake and thus reduce the likelihood of the lake freezing completely. Underwater springs and flowing water coming into the lake also contribute some crucial warmth at this time.
In the spring, the ice melts and the water is again at a constant temperature throughout the water column; in large lakes this temperature is 4o C at both the fall and spring turnover (mixing). Again, this mixing allows nutrients from the bottom to enter surface waters, and allows O2 to reach the bottom. Often a bloom (massive growth) of algae will occur at this point. The city of Akron obtains its drinking water from a lake; you can estimate when spring turnover has occurred by the taste and smell of the water, which is affected by blooms of the alga Dinobryon. Soon, increasing insolation begins the process of stratification by warming the surface waters (although strong storms will cause mixing for some time). If you can measure the levels of plant nutrients in the epilimnion, or the level of O2 in the hypolimnion, you have a pretty good idea of the limits on biological processes that will constrain those two habitats for the summer (but remember that additional plant nutrients can enter the surface waters). The process of cultural eutrophication contributes to both algal blooms in the epilimnion and oxygen deficits in the hypolimnion; such effects are much less common in oligotrophic lakes.
A lake that exhibits two periods of mixing separated by two periods of stratification is known as a dimictic lake. There are also monomictic lakes, usually in warmer climes where the lake doesn't freeze (or in some large lakes in cooler areas); polymictic lakes which mix constantly; and oligomictic lakes which are often found near the equator, remain stratified year-round, and thus rarely mix. Mixing of a lake may be complete (holomixis) or incomplete (meromixis).
Benthic sediments are often very much like the parent rock of the surrounding watershed. Often, waves or currents will sort the bottom sediments into areas of uniform sizes. The chief means of characterizing sediments is based on size:
Name |
Diameter (mm) |
Diameter (phi units) |
Boulder |
>256 |
< -8 |
Large cobble |
256 - 128 |
-8 to -7 |
Small cobble |
128 - 64 |
-7 to -6 |
Very large pebble |
64 - 32 |
-6 to -5 |
Large pebble |
32 - 16 |
-5 to -4 |
Medium pebble |
16 - 8 |
-4 to -3 |
Small pebble |
8 - 4 |
-3 to -2 |
Granule |
4 - 2 |
-2 to -1 |
Very coarse sand |
2 - 1 |
-1 to 0 |
Coarse sand |
1 - 0.5 |
0 to 1 |
Medium sand |
0.5 - 0.25 |
1 to 2 |
Fine sand |
0.25 - 0.125 |
2 to 3 |
Very fine sand |
0.125 - 0.0625 |
3 to 4 |
Coarse silt |
0.0625 - 0.03125 |
4 to 5 |
Medium silt |
0.03125 - 0.015625 |
5 to 6 |
Fine silt |
0.015625 - 0.0078125 |
6 to 7 |
Very fine silt |
0.0078125 - 0.0039063 |
7 to 8 |
Coarse clay |
0.0039063 - 0.0019531 |
8 to 9 |
Medium clay |
0.0019531 - 0.0009766 |
9 to 10 |
Fine clay |
0.0009766 - 0.0004883 |
10 to 11 |
--------------------- |
--------------------------- |
----------------------- |
Note the small size of the boulder (down to about 1 foot) in terms of what you normally consider to be a boulder! Generally, in a lake, the coarse sediments settle out near the inflows at the edges of the lake, and finer sediments will predominate in the profundal benthos.
In addition to the inorganic material, considerable organic material will settle to the bottom of lakes. This material may be modified by organisms living near the bottom and take on several forms, including gyttja or copropel. This material is largely formed by decaying plankton settling to the bottom, being eaten by bottom dwelling organisms such as Chironomus, and excreted as feces (Kopros Gr.: dung). It is gray or dark brown in color, and may appear gelatinous or as small pellets. The layer of copropel is thicker in eutrophic lakes. Sapropel forms when bottom sediments do not obtain sufficient O2; it is black in color and smells like rotten eggs due to the presences of H2S and methane. Formation of sapropel is another indication of eutrophic conditions.
Overall, several zones can be delineated in a lake (Fig. 3). The benthic habitats include the littoral zone, where enough light reaches the bottom to support plant growth, and the profundal zone, which is below the LCP and often below the thermocline as well. The habitats in the water column include the littoral zone, where plants are present, the open water area above the LCP known as the limnetic zone, and the area below the LCP which is also called the profundal zone. The epilimnion and hypolimnion are also present in stratified lakes and may or may not correspond to the neritic and profundal zones.
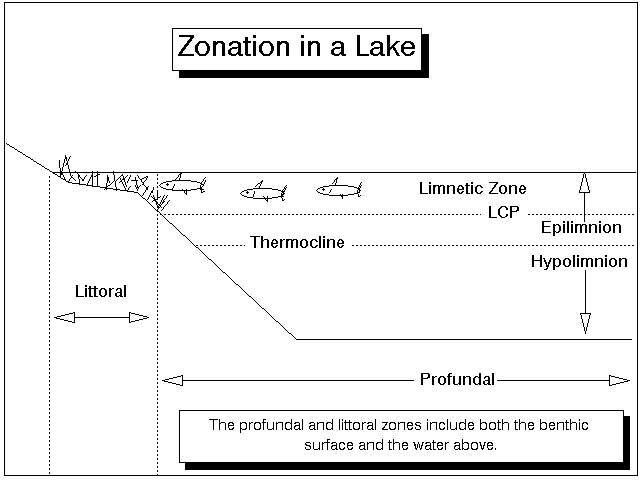
Figure 3. Habitats in a lake. The littoral zone rings the lake and includes that portion of the water column where plants emerge from the water, or that portion of the bottom where rooted plants are found. The limnetic zone is the open water above the LCP; the profundal zone is the water below the LCP and the benthic areas below. The thermocline is an area of rapid water temperature change with increasing depth; it exists only in stratified lakes and divides the water column into an upper epilimnion and lower hypolimnion. The epilimnion and hypolimnion correlate with the limnetic and profundal zones only when the thermocline is located at the LCP.
By now, you should be aware that some of the most biologically interesting habitats come at boundaries. The principle is so general that it extends to terrestrial ecosystems and even to human political systems. Mobile organisms living near boundaries can indeed have the best of both worlds. Vents, estuaries, rocky shores, all benthic habitats, the air-water interface: all of these are productive habitats located on boundaries. Before we leave the subject of habitats, let us consider a number of very small habitats often overlooked, yet extremely interesting biologically.
Temporary pools come in all sizes and shapes, but share in common the fate of being ephemeral - they dry up. Often overlooked, they may contain a diverse assembly of living things. Many of these organisms have unique strategies that allow them to survive long periods of dessication. These might include drought-resistant eggs or spores, the ability to burrow down into the mud and conserve water (found in lungfish and a number of amphibians), and the ability to actually dry out, yet come back to life when rehydrated (cryptobiosis, exhibited by tardigrades and rotifers, among others). Temporary ponds are often shallow, well-lit, predator-free, and rich in nutrients, making them favorable habitats for the organisms which can tailor their life cycles to periodic dessication and large fluctuations in salinity and temperature. Many organisms show drastically decreased life cycle times as compared to other members of their taxon which do not inhabit temporary pools. Temporary pools normally form in depressions during wet seasons, after snowmelt, during flooding, or even just after a rainfall. The nature of the basin is variable: pockets in rock, low-lying areas, holes in tree stumps, pockets formed by leaves, and human structures such as cattle troughs, tire ruts, drainage ditches, bird baths, and so on. In fact, human-created habitats may be particularly important; some of the worst mosquito pests do their best breeding in water that collects in old tires; the tires absorb heat from the sun and form warm, sheltered habitat for the mosquito larvae. The life span of temporary pools may last from hours to months. Organisms which colonize such habitats may travel arrive as resistant spores or eggs carried on the wind or by waterfowl; they may persist in resistant stages in the soil; or they may be carried in by floodwaters. Amphibians are noted for their use of temporary ponds as breeding sites; of course the adult amphibians can traverse the terrain to deposit eggs in the temporary pond.
Take a Tour of Lake Habitats Take A Tour of Wetland Habitats